金屬材料跟周邊環境間產生的電化學或者化學反應而導致材料被破壞或者使材料產生變質的現象稱為金屬材料腐蝕。金屬腐蝕產生的重要條件為所處環境中必須具有能夠讓金屬發生氧化的物質,這種物質能夠與金屬構成熱力學不穩定體系。金屬發生腐蝕的類型可以分局部腐蝕跟均勻腐蝕,其中局部腐蝕包括孔蝕、應力腐蝕、縫隙腐蝕等。對于雙相不銹鋼來講,點蝕和應力腐蝕都具有沒有明顯預兆、不易被察覺、破壞性極大的特點,是在化工生產、海洋等行業中經常遇到的問題,所以雙相不銹鋼的應力腐蝕破裂和孔(kong)蝕受到了研究者的廣泛關注。
1. 均勻(yun)腐(fu)蝕
均勻腐(fu)蝕(Uniform Corrosion)表(biao)(biao)示腐(fu)蝕環境中于(yu)(yu)金(jin)屬(shu)(shu)(shu)所(suo)有表(biao)(biao)面(mian)或者(zhe)金(jin)屬(shu)(shu)(shu)表(biao)(biao)面(mian)絕大多數區域(yu)進行的腐(fu)蝕,因而也可稱為全(quan)面(mian)腐(fu)蝕,其(qi)(qi)往(wang)往(wang)能(neng)夠導(dao)致(zhi)金(jin)屬(shu)(shu)(shu)變薄。從重(zhong)(zhong)量角度來說(shuo)(shuo),均勻腐(fu)蝕是金(jin)屬(shu)(shu)(shu)材料最大破壞(huai)程度的代表(biao)(biao),導(dao)致(zhi)的金(jin)屬(shu)(shu)(shu)損耗最為嚴重(zhong)(zhong),但是由(you)于(yu)(yu)其(qi)(qi)發生(sheng)在(zai)金(jin)屬(shu)(shu)(shu)的全(quan)部表(biao)(biao)面(mian),易于(yu)(yu)發現和控制,因而從技(ji)術層面(mian)來說(shuo)(shuo)其(qi)(qi)危(wei)害(hai)性不大。
2.點蝕
點(dian)(dian)(dian)蝕(shi)(shi)(shi)(shi)又稱小孔(kong)腐蝕(shi)(shi)(shi)(shi)、孔(kong)蝕(shi)(shi)(shi)(shi)或者點(dian)(dian)(dian)蝕(shi)(shi)(shi)(shi),是集中在金(jin)(jin)屬(shu)(shu)表(biao)面較(jiao)小區域(yu)內、能夠向金(jin)(jin)屬(shu)(shu)內部發展、直徑小而(er)深(shen)的一類腐蝕(shi)(shi)(shi)(shi)狀態。小孔(kong)腐蝕(shi)(shi)(shi)(shi)的嚴重程度一般用點(dian)(dian)(dian)蝕(shi)(shi)(shi)(shi)系(xi)數(shu)(蝕(shi)(shi)(shi)(shi)孔(kong)的最大深(shen)度和金(jin)(jin)屬(shu)(shu)平(ping)均腐蝕(shi)(shi)(shi)(shi)深(shen)度之(zhi)間的比值)表(biao)征,點(dian)(dian)(dian)蝕(shi)(shi)(shi)(shi)系(xi)數(shu)越(yue)高,點(dian)(dian)(dian)蝕(shi)(shi)(shi)(shi)產(chan)生的程度越(yue)深(shen)。當氧化劑(ji)跟(gen)鹵素離(li)子同時(shi)存在時(shi),就會導(dao)致金(jin)(jin)屬(shu)(shu)局部溶解(jie)進(jin)而(er)形成孔(kong)穴促進(jin)點(dian)(dian)(dian)蝕(shi)(shi)(shi)(shi)的產(chan)生。
a. 點蝕產(chan)生的主要條件
①. 一般情況下點蝕(shi)較容易發生(sheng)在(zai)表(biao)面(mian)(mian)具(ju)有(you)陰極性鍍(du)層或表(biao)面(mian)(mian)存在(zai)鈍(dun)化(hua)膜(mo)的金(jin)屬(shu)上。當金(jin)屬(shu)表(biao)面(mian)(mian)這(zhe)些膜(mo)的局部位置產生(sheng)破壞,裸(luo)露出的新表(biao)面(mian)(mian)(陽極)與該膜(mo)層未被破壞區(qu)域(陰極)就會形成活化(hua)-鈍(dun)化(hua)腐蝕(shi)電(dian)池(chi),進而導致腐蝕(shi)朝著(zhu)金(jin)屬(shu)內部縱深(shen)處發展促進小孔(kong)的生(sheng)成。
②. 點(dian)蝕常發生于含有特(te)殊離(li)子(zi)的腐蝕環境(jing)中(zhong),例如,雙相(xiang)不(bu)(bu)銹(xiu)鋼對(dui)鹵素離(li)子(zi)比(bi)較敏(min)感,如氯離(li)子(zi)、溴離(li)子(zi)、碘離(li)子(zi)等(deng),這些鹵素離(li)子(zi)會不(bu)(bu)均勻吸附在雙相(xiang)不(bu)(bu)銹(xiu)鋼的表面(mian),進(jin)而促進(jin)材料表面(mian)膜發生不(bu)(bu)均勻破壞。
③. 點(dian)蝕(shi)的發(fa)生存在一個臨界電(dian)位(wei),這個電(dian)位(wei)被稱為點(dian)蝕(shi)電(dian)位(wei)或(huo)者(zhe)擊穿電(dian)位(wei),一般情況下(xia)當(dang)電(dian)位(wei)高于(yu)點(dian)蝕(shi)電(dian)位(wei)時會發(fa)生點(dian)蝕(shi)。
b. 點蝕(shi)機理
點蝕的發生主要(yao)有三個階段:
①. 蝕(shi)孔成(cheng)核
鈍(dun)(dun)化(hua)(hua)膜(mo)(mo)吸附跟(gen)破(po)壞(huai)理(li)論(lun)可以用來解釋蝕(shi)(shi)孔(kong)成核(he)的(de)(de)(de)原(yuan)因。鈍(dun)(dun)化(hua)(hua)膜(mo)(mo)破(po)壞(huai)理(li)論(lun)認為:因為腐蝕(shi)(shi)性陰離(li)子半徑比(bi)較小,因而當其吸附在雙相不銹鋼表面(mian)鈍(dun)(dun)化(hua)(hua)膜(mo)(mo)上(shang)時就會(hui)很(hen)容易穿透鈍(dun)(dun)化(hua)(hua)膜(mo)(mo),進(jin)而導致“氧化(hua)(hua)膜(mo)(mo)受到(dao)污染”及促進(jin)強烈的(de)(de)(de)感應離(li)子導電(dian)的(de)(de)(de)形成,因此(ci)于(yu)一定(ding)點(dian)(dian)(dian)處該膜(mo)(mo)可以保持(chi)比(bi)較高的(de)(de)(de)電(dian)流密度,導致陽(yang)離(li)子無規(gui)律移動(dong)進(jin)而變得活躍,當溶(rong)液一膜(mo)(mo)之間(jian)(jian)的(de)(de)(de)界(jie)面(mian)電(dian)場到(dao)達某個臨界(jie)值(zhi)時就會(hui)產(chan)(chan)生(sheng)(sheng)點(dian)(dian)(dian)蝕(shi)(shi)。鈍(dun)(dun)化(hua)(hua)膜(mo)(mo)吸附理(li)論(lun)指出點(dian)(dian)(dian)蝕(shi)(shi)的(de)(de)(de)產(chan)(chan)生(sheng)(sheng)是(shi)氧跟(gen)氯離(li)子之間(jian)(jian)競爭(zheng)吸附導致的(de)(de)(de),因為當氯離(li)子取代了金(jin)(jin)(jin)屬表面(mian)氧的(de)(de)(de)吸附點(dian)(dian)(dian)后就會(hui)產(chan)(chan)生(sheng)(sheng)可溶(rong)性的(de)(de)(de)金(jin)(jin)(jin)屬-羥(qian)-氯絡合(he)物(wu),導致金(jin)(jin)(jin)屬表面(mian)膜(mo)(mo)發生(sheng)(sheng)破(po)壞(huai)進(jin)而促進(jin)了點(dian)(dian)(dian)蝕(shi)(shi)的(de)(de)(de)產(chan)(chan)生(sheng)(sheng),蝕(shi)(shi)核(he)產(chan)(chan)生(sheng)(sheng)以后這個點(dian)(dian)(dian)依然有再(zai)(zai)鈍(dun)(dun)化(hua)(hua)的(de)(de)(de)能力(li),如果該點(dian)(dian)(dian)的(de)(de)(de)再(zai)(zai)鈍(dun)(dun)化(hua)(hua)能力(li)很(hen)強,蝕(shi)(shi)核(he)就不會(hui)繼續變大。小蝕(shi)(shi)孔(kong)表現為開放式(shi)的(de)(de)(de)狀態(tai),在晶界(jie)上(shang)碳化(hua)(hua)物(wu)沉(chen)積、金(jin)(jin)(jin)屬內部硫化(hua)(hua)物(wu)夾(jia)雜(za)及晶界(jie)、金(jin)(jin)(jin)屬表面(mian)的(de)(de)(de)劃(hua)痕、位錯(cuo)露頭等缺(que)陷處更容易形成蝕(shi)(shi)核(he)。
②. 蝕孔生長(chang)階(jie)段
蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)生(sheng)(sheng)(sheng)成之后,孔(kong)(kong)(kong)(kong)(kong)蝕(shi)(shi)(shi)(shi)的(de)(de)(de)(de)發展是(shi)十分(fen)迅速的(de)(de)(de)(de),一般用自(zi)催化(hua)過程來解(jie)(jie)釋蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)的(de)(de)(de)(de)生(sheng)(sheng)(sheng)長(chang),如圖所示。雙(shuang)相不(bu)銹鋼在存在氯離子(zi)的(de)(de)(de)(de)溶(rong)液中,陰極處會(hui)(hui)發生(sheng)(sheng)(sheng)吸氧反應使孔(kong)(kong)(kong)(kong)(kong)內氧的(de)(de)(de)(de)濃(nong)度(du)降(jiang)低,然(ran)而(er)孔(kong)(kong)(kong)(kong)(kong)外(wai)(wai)的(de)(de)(de)(de)氧、氧濃(nong)度(du)依(yi)然(ran)較高(gao),所以孔(kong)(kong)(kong)(kong)(kong)內外(wai)(wai)的(de)(de)(de)(de)“供(gong)養差(cha)異電池(chi)”較容易(yi)形成。在孔(kong)(kong)(kong)(kong)(kong)內金(jin)屬(shu)離子(zi)連續變(bian)多的(de)(de)(de)(de)情(qing)況下,蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)外(wai)(wai)的(de)(de)(de)(de)氯離子(zi)會(hui)(hui)向(xiang)孔(kong)(kong)(kong)(kong)(kong)內移動從而(er)達到能夠維(wei)持(chi)溶(rong)液電中性的(de)(de)(de)(de)目的(de)(de)(de)(de)。此(ci)外(wai)(wai),孔(kong)(kong)(kong)(kong)(kong)內金(jin)屬(shu)離子(zi)漸(jian)漸(jian)變(bian)多并發生(sheng)(sheng)(sheng)水(shui)解(jie)(jie)導致蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)內部(bu)(bu)(bu)H+濃(nong)度(du)不(bu)斷升高(gao),這時蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)內部(bu)(bu)(bu)酸(suan)化(hua)就會(hui)(hui)造成孔(kong)(kong)(kong)(kong)(kong)內的(de)(de)(de)(de)金(jin)屬(shu)材料表現為活化(hua)溶(rong)解(jie)(jie)狀態(tai);而(er)蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)外(wai)(wai)部(bu)(bu)(bu)的(de)(de)(de)(de)表面(mian)膜由于依(yi)然(ran)保持(chi)鈍(dun)態(tai)進而(er)形成了(le)活化(hua)(蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)內)-鈍(dun)化(hua)(蝕(shi)(shi)(shi)(shi)孔(kong)(kong)(kong)(kong)(kong)外(wai)(wai))電池(chi),促(cu)使金(jin)屬(shu)不(bu)斷產生(sheng)(sheng)(sheng)溶(rong)解(jie)(jie),進而(er)導致孔(kong)(kong)(kong)(kong)(kong)蝕(shi)(shi)(shi)(shi)按照自(zi)催化(hua)的(de)(de)(de)(de)過程繼續發展,促(cu)使腐蝕(shi)(shi)(shi)(shi)產生(sheng)(sheng)(sheng)。
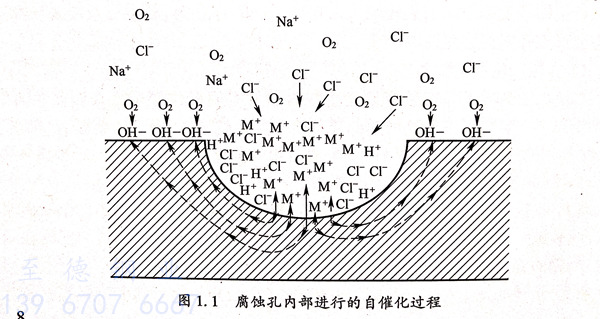
③. 蝕孔再(zai)鈍(dun)化階段
蝕孔(kong)(kong)(kong)內(nei)金(jin)屬發(fa)(fa)生(sheng)的(de)再(zai)(zai)鈍化(hua)(hua)(hua)(hua)會(hui)導致孔(kong)(kong)(kong)蝕進行到某個深度之后就不會(hui)繼(ji)續(xu)進行了。造成(cheng)蝕孔(kong)(kong)(kong)再(zai)(zai)鈍化(hua)(hua)(hua)(hua)的(de)成(cheng)因有三種:一是(shi)(shi)蝕孔(kong)(kong)(kong)內(nei)電(dian)位(wei)朝(chao)著負方(fang)向移動至小于保護電(dian)位(wei)(E.)時金(jin)屬就會(hui)進入到鈍化(hua)(hua)(hua)(hua)區域,金(jin)屬再(zai)(zai)鈍化(hua)(hua)(hua)(hua)的(de)產生(sheng)也可(ke)能是(shi)(shi)周(zhou)邊區域的(de)孔(kong)(kong)(kong)蝕劇(ju)烈(lie)發(fa)(fa)展或(huo)腐蝕介質的(de)氧化(hua)(hua)(hua)(hua)還原(yuan)電(dian)位(wei)降(jiang)低所造成(cheng)的(de);二(er)是(shi)(shi)金(jin)屬表面鈍化(hua)(hua)(hua)(hua)膜(mo)比較(jiao)脆(cui)弱的(de)區域被消(xiao)除(chu),如夾雜物及(ji)晶間沉淀(dian),金(jin)屬的(de)再(zai)(zai)鈍化(hua)(hua)(hua)(hua)有可(ke)能在其被消(xiao)除(chu)之后而(er)產生(sheng);三是(shi)(shi)蝕孔(kong)(kong)(kong)內(nei)部(bu)的(de)歐姆電(dian)壓會(hui)隨著孔(kong)(kong)(kong)蝕的(de)生(sheng)長而(er)漸(jian)漸(jian)變大,導致蝕孔(kong)(kong)(kong)內(nei)部(bu)的(de)電(dian)位(wei)轉移到鈍化(hua)(hua)(hua)(hua)區域,從而(er)使(shi)金(jin)屬發(fa)(fa)生(sheng)再(zai)(zai)鈍化(hua)(hua)(hua)(hua)現(xian)象。
3. 縫隙腐(fu)蝕
金(jin)(jin)屬跟(gen)非金(jin)(jin)屬或者金(jin)(jin)屬跟(gen)金(jin)(jin)屬表面具有縫(feng)(feng)隙(xi),并(bing)且(qie)腐(fu)蝕(shi)(shi)介(jie)質也同時存在時產(chan)生(sheng)的(de)(de)腐(fu)蝕(shi)(shi)稱為縫(feng)(feng)隙(xi)腐(fu)蝕(shi)(shi)。通常情況下,縫(feng)(feng)隙(xi)腐(fu)蝕(shi)(shi)發生(sheng)的(de)(de)縫(feng)(feng)寬(kuan)為0.025~0.1mm,這個寬(kuan)度能夠讓電(dian)解質溶(rong)液進(jin)入,進(jin)而(er)導致縫(feng)(feng)隙(xi)內(nei)部(bu)跟(gen)外部(bu)的(de)(de)金(jin)(jin)屬組(zu)成短路電(dian)池發生(sheng)強(qiang)烈的(de)(de)腐(fu)蝕(shi)(shi),并(bing)且(qie)縫(feng)(feng)隙(xi)內(nei)部(bu)金(jin)(jin)屬作為陽極(ji)(ji),縫(feng)(feng)隙(xi)外部(bu)金(jin)(jin)屬作為陰極(ji)(ji)。其擴展機(ji)理(li)與點(dian)蝕(shi)(shi)類似都是自(zi)催化(hua)過程,但是始發的(de)(de)機(ji)理(li)是不一樣(yang)的(de)(de),此外就同一種金(jin)(jin)屬而(er)言(yan)相,對于點(dian)蝕(shi)(shi)較(jiao)易產(chan)生(sheng)縫(feng)(feng)隙(xi)腐(fu)蝕(shi)(shi)。
4. 晶間腐蝕
在特定的(de)(de)(de)(de)腐蝕(shi)(shi)環境中,沿(yan)著或(huo)者緊挨(ai)著金屬晶(jing)(jing)(jing)粒邊界(jie)產生(sheng)的(de)(de)(de)(de)腐蝕(shi)(shi)稱(cheng)為晶(jing)(jing)(jing)間(jian)腐蝕(shi)(shi)。晶(jing)(jing)(jing)間(jian)腐蝕(shi)(shi)是(shi)一(yi)種局部(bu)破壞(huai)現(xian)象,可以讓(rang)金屬晶(jing)(jing)(jing)粒之(zhi)間(jian)的(de)(de)(de)(de)結合力消失(shi)。當金屬發生(sheng)晶(jing)(jing)(jing)間(jian)腐蝕(shi)(shi)并且有應力對其(qi)進行(xing)作用時,金屬的(de)(de)(de)(de)強度(du)就會(hui)幾乎全(quan)部(bu)喪失(shi)、會(hui)沿(yan)晶(jing)(jing)(jing)界(jie)發生(sheng)斷(duan)裂,但是(shi)金屬發生(sheng)的(de)(de)(de)(de)這種破壞(huai)是(shi)不(bu)易被觀察(cha)到(dao)的(de)(de)(de)(de),因為在其(qi)表面依然會(hui)呈現(xian)出(chu)一(yi)定的(de)(de)(de)(de)金屬光澤,所(suo)以晶(jing)(jing)(jing)間(jian)腐蝕(shi)(shi)是(shi)一(yi)類比較(jiao)危險的(de)(de)(de)(de)腐蝕(shi)(shi)。
5. 應力(li)腐蝕
應(ying)力(li)(li)腐(fu)蝕(shi)(shi)破裂(SCC)是指在腐(fu)蝕(shi)(shi)介質和拉伸應(ying)力(li)(li)兩者共同(tong)影響下(xia)造成金屬發生脆性斷裂的(de)現(xian)象。材料與介質的(de)匹(pi)配(pei)性是應(ying)力(li)(li)腐(fu)蝕(shi)(shi)破裂的(de)一(yi)個主(zhu)要特點之一(yi)。應(ying)力(li)(li)腐(fu)蝕(shi)(shi)破裂是在無顯(xian)著征兆的(de)情況下(xia)突然發生的(de),因而破壞性及危(wei)險性極大,在不銹鋼腐(fu)蝕(shi)(shi)破壞形(xing)式中,應(ying)力(li)(li)腐(fu)蝕(shi)(shi)占(zhan)20%以(yi)上,因此(ci),雙(shuang)相不銹鋼的(de)應(ying)力(li)(li)腐(fu)蝕(shi)(shi)是一(yi)個很重要的(de)實際問題。



