圖3.7(a)~(d)為(wei)(wei)(wei)(wei)鐵(tie)(tie)素體(ti)(ti)(ti)和奧(ao)氏(shi)體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr、Mo、Ni元(yuan)素含(han)(han)(han)量(liang)(liang)與固(gu)(gu)(gu)溶(rong)(rong)(rong)溫(wen)(wen)度(du)(du)(du)的(de)(de)(de)(de)關系圖。圖3.7(a)~(d)證(zheng)實了鐵(tie)(tie)素體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr和Mo含(han)(han)(han)量(liang)(liang)更(geng)高(gao)(gao),而(er)奧(ao)氏(shi)體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Ni和Mn含(han)(han)(han)量(liang)(liang)更(geng)高(gao)(gao)。從圖中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)可(ke)(ke)看出,鐵(tie)(tie)素體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr含(han)(han)(han)量(liang)(liang)為(wei)(wei)(wei)(wei)23.77%~25.16%,比(bi)(bi)奧(ao)氏(shi)體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)Cr含(han)(han)(han)量(liang)(liang)高(gao)(gao)2%左右;鐵(tie)(tie)素體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)Mo含(han)(han)(han)量(liang)(liang)為(wei)(wei)(wei)(wei)3.86%~4.37%,比(bi)(bi)奧(ao)氏(shi)體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Mo含(han)(han)(han)量(liang)(liang)高(gao)(gao)1.7%左右;奧(ao)氏(shi)體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Ni含(han)(han)(han)量(liang)(liang)為(wei)(wei)(wei)(wei)5.42%、6.7%,比(bi)(bi)鐵(tie)(tie)素體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Ni含(han)(han)(han)量(liang)(liang)高(gao)(gao)2%;當固(gu)(gu)(gu)溶(rong)(rong)(rong)處理溫(wen)(wen)度(du)(du)(du)為(wei)(wei)(wei)(wei)1050℃時(shi),鐵(tie)(tie)素體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr、Mo和Ni含(han)(han)(han)量(liang)(liang)分別為(wei)(wei)(wei)(wei)23.77%、3.97%、4.24%,奧(ao)氏(shi)體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr、Mo和Ni含(han)(han)(han)量(liang)(liang)分別為(wei)(wei)(wei)(wei)23.53%、2.63%、5.42%.可(ke)(ke)見(jian)在1050℃溫(wen)(wen)度(du)(du)(du)下(xia)進(jin)行固(gu)(gu)(gu)溶(rong)(rong)(rong)時(shi),兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr、Mo、Ni含(han)(han)(han)量(liang)(liang)差異(yi)最(zui)小,此時(shi)鐵(tie)(tie)素體(ti)(ti)(ti)含(han)(han)(han)量(liang)(liang)為(wei)(wei)(wei)(wei)51.9%,奧(ao)氏(shi)體(ti)(ti)(ti)含(han)(han)(han)量(liang)(liang)為(wei)(wei)(wei)(wei)48.1%.當固(gu)(gu)(gu)溶(rong)(rong)(rong)溫(wen)(wen)度(du)(du)(du)改(gai)變時(shi),鐵(tie)(tie)素體(ti)(ti)(ti)/奧(ao)氏(shi)體(ti)(ti)(ti)兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)比(bi)(bi)例相(xiang)(xiang)(xiang)(xiang)(xiang)差變大(da)(da),且兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr、Mo、Ni含(han)(han)(han)量(liang)(liang)差異(yi)也(ye)變大(da)(da)。當固(gu)(gu)(gu)溶(rong)(rong)(rong)溫(wen)(wen)度(du)(du)(du)為(wei)(wei)(wei)(wei)1000℃,兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)Cr為(wei)(wei)(wei)(wei)3.69%、Mo為(wei)(wei)(wei)(wei)1.51%、Ni為(wei)(wei)(wei)(wei)3.37%;當固(gu)(gu)(gu)溶(rong)(rong)(rong)溫(wen)(wen)度(du)(du)(du)為(wei)(wei)(wei)(wei)1150℃時(shi),兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)Cr為(wei)(wei)(wei)(wei)2.29%、Mo為(wei)(wei)(wei)(wei)1.34%、Ni為(wei)(wei)(wei)(wei)2.09%,可(ke)(ke)見(jian)1000℃固(gu)(gu)(gu)溶(rong)(rong)(rong)試樣的(de)(de)(de)(de)兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)的(de)(de)(de)(de)Cr、Mo、Ni元(yuan)素含(han)(han)(han)量(liang)(liang)差大(da)(da)于1150℃固(gu)(gu)(gu)溶(rong)(rong)(rong)試樣的(de)(de)(de)(de)兩(liang)相(xiang)(xiang)(xiang)(xiang)(xiang)中(zhong)(zhong)(zhong)(zhong)(zhong)(zhong)Cr、Mo、Ni元(yuan)素含(han)(han)(han)量(liang)(liang)差。
由公式“PREN.=[Cr]+3.3[Mo]”計算鐵素體(ti)的PREN值,由公式“PREN,=[Cr]+3.3[Mo]+16[N]-[Mn]”計算奧(ao)氏(shi)體(ti)的PREN值,可(ke)得(de)PREN值與固溶溫(wen)(wen)度的關系圖,如圖3.7(e)所示(shi)。從圖3.7(e)可(ke)看出,在(zai)不同固溶狀態(tai)下,兩相(xiang)的PREN值有所不同,但PRENa>PRENy.當固溶溫(wen)(wen)度為1050℃時,PREN.最(zui)小(xiao)、PREN,最(zui)大,分別(bie)為36.9和30.6,兩者相(xiang)差(cha)最(zui)小(xiao)。
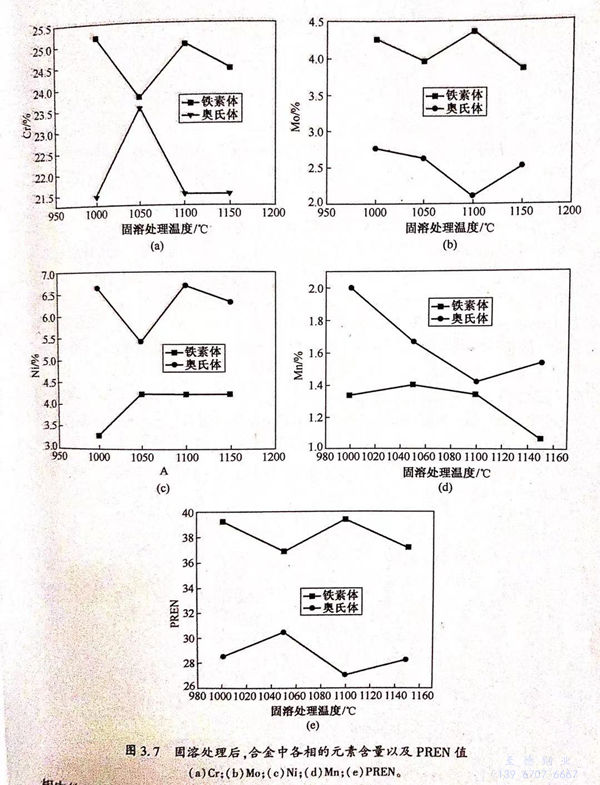
圖3.8為(wei)不(bu)同固(gu)溶試樣的(de)(de)極化(hua)曲線(xian)。可(ke)見,在自(zi)腐蝕(shi)電(dian)(dian)(dian)位(wei)下,材料(liao)開始發生鈍化(hua);當極化(hua)電(dian)(dian)(dian)位(wei)升高到一(yi)定值時,不(bu)同固(gu)溶的(de)(de)材料(liao)都(dou)發生點(dian)(dian)(dian)蝕(shi),電(dian)(dian)(dian)流(liu)(liu)密(mi)度急劇(ju)增大。根(gen)據GB 4334.9-1984中(zhong)電(dian)(dian)(dian)流(liu)(liu)密(mi)度為(wei)0.1mA/c㎡所(suo)(suo)對應的(de)(de)電(dian)(dian)(dian)位(wei)為(wei)點(dian)(dian)(dian)蝕(shi)電(dian)(dian)(dian)位(wei)和Tafel擬合,分別得到點(dian)(dian)(dian)蝕(shi)電(dian)(dian)(dian)位(wei)、自(zi)腐蝕(shi)電(dian)(dian)(dian)流(liu)(liu)密(mi)度與固(gu)溶溫度的(de)(de)關系(xi)圖,如圖3.11(a)所(suo)(suo)示。
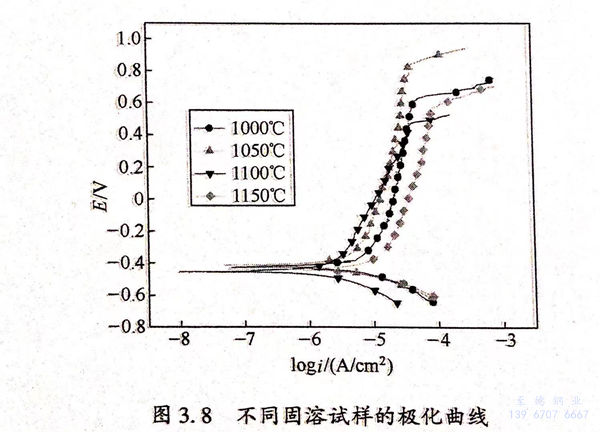
圖(tu)3.9(a)、(b)分(fen)別為不同固溶(rong)(rong)試(shi)樣的Nyquist 圖(tu)和(he)Bode圖(tu)。從圖(tu)3.9(a)可見,試(shi)樣在(zai)低(di)頻(pin)和(he)高(gao)頻(pin)區分(fen)別存(cun)在(zai)一(yi)個(ge)容抗(kang)(kang)弧。從圖(tu)3.9(b)可見,試(shi)樣在(zai)低(di)頻(pin)和(he)高(gao)頻(pin)處分(fen)別存(cun)在(zai)一(yi)個(ge)時(shi)間常(chang)數(shu)。根(gen)據曹楚南(nan)的《電化學阻抗(kang)(kang)譜導(dao)論》可知。雙(shuang)相不銹鋼在(zai)NaCl溶(rong)(rong)液(ye)中(zhong)的阻抗(kang)(kang)譜中(zhong)存(cun)在(zai)兩個(ge)時(shi)間常(chang)數(shu),常(chang)以圖(tu)3.10所示的等效電路(R1為電荷(he)轉移(yi)電阻,R2為鈍化膜電阻)進行阻抗(kang)(kang)擬(ni)合(he)。經阻抗(kang)(kang)擬(ni)合(he),得到鈍化膜電阻、電荷(he)轉移(yi)電阻與固溶(rong)(rong)處理溫(wen)度的關系圖(tu),如(ru)圖(tu)3.11(b)所示。
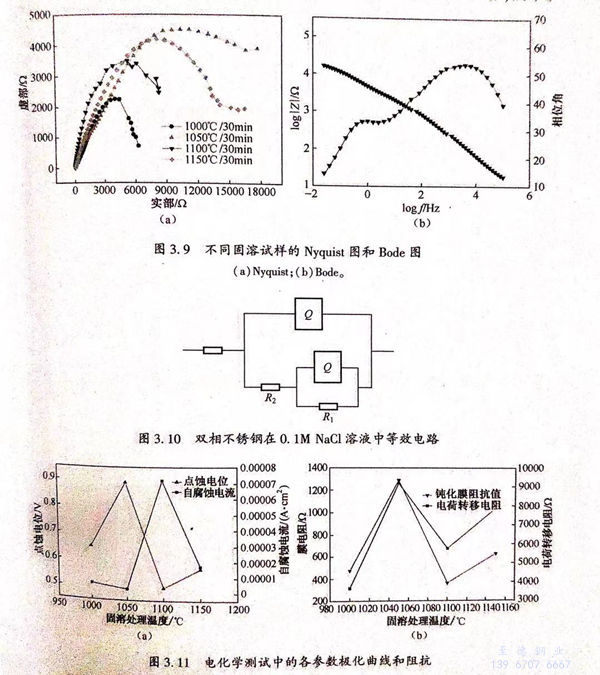
從圖3.11(a)可見,1050℃固溶試樣的點蝕電位最高,約為0.89V,且1050℃固溶試樣的自腐蝕電流密度最低,約為2.34×10-6A/c㎡,說明該狀態下試樣的耐點蝕性能最好。由圖3.11(b)可知,1050℃固溶試樣的鈍化膜電阻值最高,約為1300Ω,且其電荷轉移電阻值也最高,約為9500Ω,說明該狀態下試樣的鈍化膜較穩定,耐點蝕性能較好。
圖3.12為不同固溶的2205雙相不(bu)銹鋼極化后的點蝕形貌圖,圖中黑色為蝕坑,深色為鐵素體,淡色為奧氏體。從圖3.12可見,四種固溶試樣的點蝕都發生于鐵素體與奧氏體相界面處,且易向鐵素體相中長大。當固溶溫度為1050℃時,試樣的點蝕尺寸最小,點蝕敏感性最低。

通(tong)(tong)過(guo)計算得到不(bu)(bu)(bu)同(tong)相(xiang)比(bi)例(li)下的鐵(tie)素(su)體PREN值(zhi)(zhi)(zhi)(zhi)和奧氏(shi)體PREN值(zhi)(zhi)(zhi)(zhi),可(ke)(ke)見(jian)不(bu)(bu)(bu)同(tong)相(xiang)比(bi)例(li)下的PREN.都大于PREN,.雙相(xiang)不(bu)(bu)(bu)銹鋼(gang)(gang)的耐(nai)點(dian)(dian)蝕(shi)(shi)性(xing)(xing)能(neng)可(ke)(ke)通(tong)(tong)過(guo)點(dian)(dian)蝕(shi)(shi)電(dian)位衡量。點(dian)(dian)蝕(shi)(shi)電(dian)位越(yue)(yue)(yue)高(gao),耐(nai)點(dian)(dian)獨性(xing)(xing)能(neng)越(yue)(yue)(yue)好(hao)。前(qian)人認為(wei)雙相(xiang)不(bu)(bu)(bu)銹鋼(gang)(gang)的耐(nai)點(dian)(dian)蝕(shi)(shi)性(xing)(xing)能(neng)由B能(neng)N值(zhi)(zhi)(zhi)(zhi)較區(qu)的相(xiang)決定,且PEN值(zhi)(zhi)(zhi)(zhi)越(yue)(yue)(yue)高(gao),耐(nai)點(dian)(dian)蝕(shi)(shi)性(xing)(xing)能(neng)越(yue)(yue)(yue)好(hao),從(cong)圖3.71e)可(ke)(ke)矩,不(bu)(bu)(bu)同(tong)海溶試(shi)樣的PREN.都大于PREN,,當固(gu)溶溫度為(wei)1050℃時,PHEN,最大,材料的耐(nai)點(dian)(dian)蝕(shi)(shi)性(xing)(xing)能(neng)應最好(hao)。從(cong)圖3.11(a)、(b)可(ke)(ke)知(zhi),1050℃固(gu)溶試(shi)樣的點(dian)(dian)蝕(shi)(shi)電(dian)位最高(gao),鈍化膜阻抗值(zhi)(zhi)(zhi)(zhi)最大,電(dian)荷(he)轉移(yi)電(dian)阻值(zhi)(zhi)(zhi)(zhi)最商;且從(cong)圖3.12(b)可(ke)(ke)見(jian),1050℃固(gu)溶試(shi)樣的點(dian)(dian)蝕(shi)(shi)坑尺寸最小,表(biao)現出最好(hao)的耐(nai)點(dian)(dian)蝕(shi)(shi)性(xing)(xing)能(neng)。綜上(shang)可(ke)(ke)知(zhi),雙相(xiang)不(bu)(bu)(bu)銹鋼(gang)(gang)的耐(nai)點(dian)(dian)蝕(shi)(shi)性(xing)(xing)能(neng)由 PREN 值(zhi)(zhi)(zhi)(zhi)較小相(xiang)決定的理論是(shi)有實(shi)驗依據的。
從(cong)圖(tu)(tu)3.12(a)~(d)可(ke)見(jian),在不同(tong)固溶狀態下,鐵(tie)素體相(xiang)都(dou)更易發生點(dian)蝕(shi);而從(cong)圖(tu)(tu)3.7(e)可(ke)見(jian),在不同(tong)固溶狀態下,PREN.都(dou)大(da)于PREN,,鐵(tie)素體的耐(nai)點(dian)蝕(shi)性能應優(you)于奧(ao)氏(shi)體相(xiang),可(ke)見(jian)兩者存在矛(mao)盾。
隨(sui)固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)溫度(du)變(bian)化(hua),雙相(xiang)(xiang)(xiang)(xiang)不銹(xiu)鋼(gang)中(zhong)的(de)(de)(de)鐵(tie)素(su)(su)體(ti)(ti)(ti)和奧氏體(ti)(ti)(ti)相(xiang)(xiang)(xiang)(xiang)的(de)(de)(de)比例改變(bian),且(qie)兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)形態也發(fa)(fa)生變(bian)化(hua)。Cr是鐵(tie)素(su)(su)體(ti)(ti)(ti)形成(cheng)(cheng)元素(su)(su),可(ke)(ke)(ke)提高材(cai)(cai)(cai)料(liao)(liao)的(de)(de)(de)耐(nai)蝕(shi)(shi)性(xing)(xing)能(neng);Mo是鐵(tie)素(su)(su)體(ti)(ti)(ti)形成(cheng)(cheng)元素(su)(su),可(ke)(ke)(ke)提高點(dian)蝕(shi)(shi)電(dian)位,降低腐(fu)(fu)蝕(shi)(shi)速率;Ni是奧氏體(ti)(ti)(ti)形成(cheng)(cheng)元素(su)(su),可(ke)(ke)(ke)維持兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)平衡(heng),提高耐(nai)蝕(shi)(shi)性(xing)(xing)能(neng)。并(bing)且(qie)材(cai)(cai)(cai)料(liao)(liao)中(zhong)存(cun)在一定量的(de)(de)(de)N,其為(wei)奧氏體(ti)(ti)(ti)形成(cheng)(cheng)元素(su)(su),提高局(ju)部腐(fu)(fu)蝕(shi)(shi)抗力。從圖(tu)3.7(a)~(c)可(ke)(ke)(ke)見,隨(sui)固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)溫度(du)變(bian)化(hua),兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)中(zhong)的(de)(de)(de)Cr、Mo、Ni元素(su)(su)含(han)量發(fa)(fa)生變(bian)化(hua)。由(you)于(yu)(yu)Cr、Mo、Ni元素(su)(su)之間(jian)的(de)(de)(de)腐(fu)(fu)蝕(shi)(shi)電(dian)位存(cun)在差(cha)異,勢必造成(cheng)(cheng)兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)之間(jian)存(cun)在電(dian)化(hua)學差(cha)異,使腐(fu)(fu)蝕(shi)(shi)更易發(fa)(fa)生。從圖(tu)3.7(e)可(ke)(ke)(ke)知,不同固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)狀(zhuang)態下材(cai)(cai)(cai)料(liao)(liao)的(de)(de)(de)PREN,大小關系為(wei)1050℃>1000℃>1150℃>1100℃,因(yin)此(ci)(ci)(ci)根據前人的(de)(de)(de)研究(jiu),不同固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)態材(cai)(cai)(cai)料(liao)(liao)的(de)(de)(de)耐(nai)點(dian)蝕(shi)(shi)性(xing)(xing)能(neng)優劣關系應為(wei):1050℃優于(yu)(yu)1000℃優于(yu)(yu)1150℃.當固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)溫度(du)為(wei)1050℃時,PREN,值(zhi)較大,兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)中(zhong)的(de)(de)(de)Cr、Mo、Ni元素(su)(su)含(han)量差(cha)異最(zui)小,材(cai)(cai)(cai)料(liao)(liao)的(de)(de)(de)點(dian)蝕(shi)(shi)坑(keng)較小,材(cai)(cai)(cai)料(liao)(liao)的(de)(de)(de)耐(nai)點(dian)蝕(shi)(shi)性(xing)(xing)能(neng)最(zui)優。1000℃固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)試樣的(de)(de)(de)點(dian)蝕(shi)(shi)坑(keng)尺寸(cun)大于(yu)(yu)1150℃固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)試樣的(de)(de)(de)點(dian)蝕(shi)(shi)坑(keng)尺寸(cun),因(yin)此(ci)(ci)(ci),前者(zhe)的(de)(de)(de)耐(nai)點(dian)蝕(shi)(shi)性(xing)(xing)能(neng)劣于(yu)(yu)后者(zhe)的(de)(de)(de)耐(nai)點(dian)蝕(shi)(shi)性(xing)(xing)能(neng)。由(you)此(ci)(ci)(ci)可(ke)(ke)(ke)見,雙相(xiang)(xiang)(xiang)(xiang)不銹(xiu)鋼(gang)中(zhong)的(de)(de)(de)相(xiang)(xiang)(xiang)(xiang)腐(fu)(fu)蝕(shi)(shi)不能(neng)僅僅由(you)PREN值(zhi)來解釋。由(you)圖(tu)3.7(a)~(c)可(ke)(ke)(ke)知,1000℃固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)試樣的(de)(de)(de)兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)中(zhong)的(de)(de)(de)Cr、Mo、Ni元素(su)(su)含(han)量差(cha)大于(yu)(yu)1150℃固(gu)(gu)(gu)溶(rong)(rong)(rong)(rong)試樣的(de)(de)(de)兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)中(zhong)的(de)(de)(de)Gr、Mo、Ni元素(su)(su)含(han)量差(cha),因(yin)此(ci)(ci)(ci),雙相(xiang)(xiang)(xiang)(xiang)不銹(xiu)鋼(gang)中(zhong)的(de)(de)(de)相(xiang)(xiang)(xiang)(xiang)腐(fu)(fu)蝕(shi)(shi)還(huan)與(yu)兩(liang)(liang)相(xiang)(xiang)(xiang)(xiang)中(zhong)的(de)(de)(de)元素(su)(su)分布有關,并(bing)且(qie)還(huan)需考慮到(dao)材(cai)(cai)(cai)料(liao)(liao)中(zhong)點(dian)蝕(shi)(shi)敏感性(xing)(xing)較強的(de)(de)(de)區域(yu),如晶界和相(xiang)(xiang)(xiang)(xiang)界。
點蝕是一種局(ju)部腐蝕現象,是由氯離子破壞鈍化膜而導致的。點蝕產生后,蝕坑處的基體被暴露在溶液中,導致材料進一步發生腐蝕,蝕坑長大。蝕坑前長大速率由材料的均勻溶解速率決定,溶解越快,蝕坑長大速度越大。因此,雙相不銹鋼的耐點蝕性能由兩部分構成:控制點蝕萌生和控制蝕坑長大的能力。本書中的點蝕電位意味著點蝕已長大。影響點蝕萌生的因素不僅包括PREN 值,還包括兩相中的元素分布和兩相的比例。從本書的分析可見,點蝕的長大與元素分布有關,兩相中的元素分布越均勻,蝕坑的長大速度越慢。因此在1050℃固溶狀態下,兩相中的元素分布最均勻,PREN,最高,合金的點蝕電位最高,材料的耐點蝕性能最好。而1000℃固溶試樣兩相中的元素分布不均勻,導致點蝕電位較低,點蝕坑尺寸較大,降低材料的耐點蝕性能。
影響雙(shuang)相(xiang)(xiang)不(bu)銹鋼相(xiang)(xiang)腐蝕的(de)因(yin)素不(bu)僅包(bao)括PREN值(zhi),還包(bao)括各(ge)相(xiang)(xiang)中的(de)元素分布(bu)和兩相(xiang)(xiang)比例(li),并且三者互相(xiang)(xiang)影響、關系(xi)復雜。因(yin)此,不(bu)能(neng)僅憑(ping)PREN值(zhi)的(de)大小來判斷(duan)耐點蝕能(neng)力(li),應該綜合考慮(lv)各(ge)因(yin)素的(de)影響。



