一、現象
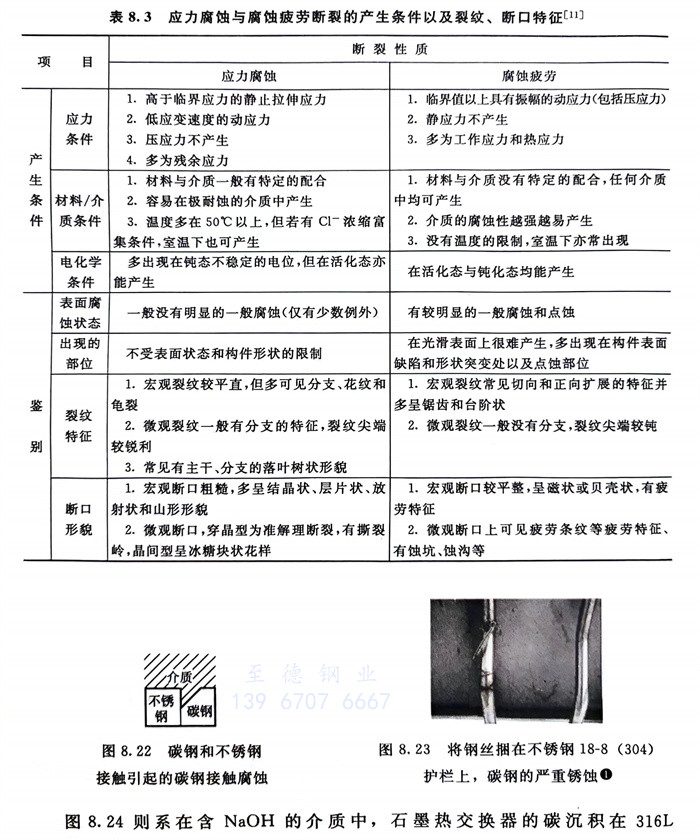
在電解質溶液中(包括潮濕大氣),碳鋼管若與不銹鋼管相接觸,碳鋼就會迅速產生腐蝕(示意圖見圖8.22),實際例子見圖8.23。后者系城市中用鋼(鐵)絲捆在18-8(304)不銹鋼護欄上所導致的鋼絲的嚴重銹蝕(鋼絲銹蝕還可誘發不銹鋼護欄的腐蝕)。
圖8.24則系在含NaOH的介質中,石墨熱交換器的碳沉積在316L(022Cr17Ni12Mo2)不銹鋼擋板上而引起的316L不銹鋼管的腐蝕。

二、原因
兩種金(jin)(jin)(jin)(jin)屬在腐(fu)(fu)(fu)蝕(shi)(shi)(shi)介質中,由于(yu)(yu)二者的(de)電(dian)(dian)(dian)(dian)極(ji)(ji)電(dian)(dian)(dian)(dian)位(wei)不(bu)(bu)(bu)同,電(dian)(dian)(dian)(dian)位(wei)較正金(jin)(jin)(jin)(jin)屬為(wei)陰(yin)極(ji)(ji),電(dian)(dian)(dian)(dian)位(wei)較負的(de)金(jin)(jin)(jin)(jin)屬為(wei)陽極(ji)(ji),當(dang)兩種金(jin)(jin)(jin)(jin)屬接觸(chu)(chu)時,它們之間(jian)就會有電(dian)(dian)(dian)(dian)流產生,出現電(dian)(dian)(dian)(dian)化學腐(fu)(fu)(fu)蝕(shi)(shi)(shi)(實際上是形(xing)成(cheng)了宏觀(guan)電(dian)(dian)(dian)(dian)池)。此時作(zuo)為(wei)陽極(ji)(ji)的(de)金(jin)(jin)(jin)(jin)屬,其(qi)腐(fu)(fu)(fu)蝕(shi)(shi)(shi)速(su)度就會提高(gao)(加速(su)腐(fu)(fu)(fu)蝕(shi)(shi)(shi)),而(er)(er)(er)作(zuo)為(wei)陰(yin)極(ji)(ji)的(de)金(jin)(jin)(jin)(jin)屬其(qi)腐(fu)(fu)(fu)蝕(shi)(shi)(shi)速(su)度就會降低(di)(di)(受到保護(hu)),從而(er)(er)(er)形(xing)成(cheng)異金(jin)(jin)(jin)(jin)屬接觸(chu)(chu)(電(dian)(dian)(dian)(dian)偶)腐(fu)(fu)(fu)蝕(shi)(shi)(shi)。前述(shu)兩例(li),前者舉例(li)表明,碳鋼(gang)的(de)電(dian)(dian)(dian)(dian)極(ji)(ji)電(dian)(dian)(dian)(dian)位(wei)遠(yuan)低(di)(di)于(yu)(yu)不(bu)(bu)(bu)銹鋼(gang),而(er)(er)(er)后者則系碳(石墨)的(de)電(dian)(dian)(dian)(dian)極(ji)(ji)電(dian)(dian)(dian)(dian)位(wei)又遠(yuan)較316L不(bu)(bu)(bu)銹鋼(gang)為(wei)正,從而(er)(er)(er)產生了異金(jin)(jin)(jin)(jin)屬接觸(chu)(chu)腐(fu)(fu)(fu)蝕(shi)(shi)(shi)所(suo)致。
三、防止措施(shi)
在濕(shi)態(tai)腐蝕環境中,當(dang)不(bu)銹(xiu)(xiu)鋼管與其他金(jin)屬(shu)相(xiang)接觸時,要(yao)考慮它們之間的(de)電(dian)(dian)極電(dian)(dian)位是否存在顯著的(de)差(cha)異。不(bu)要(yao)選用與不(bu)銹(xiu)(xiu)鋼相(xiang)比較電(dian)(dian)極電(dian)(dian)位相(xiang)差(cha)過(guo)大的(de)金(jin)屬(shu)。



